Medical Devices
Your specialist for medical devices in gynecology
One of the EurimGroup's business areas is the manufacture and distribution of intrauterine devices. Since 1998, EurimPharm has been developing and producing its own generic line of copper intrauterine devices through its Belgian subsidiary, a long-standing manufacturer of copper-containing intrauterine devices (IUDs), which is successfully marketed in numerous countries. More than 30 years of know-how, ISO certification and many years of market establishment guarantee the very highest quality.
- EurimPharm's objective is to offer high quality products at competitive prices.
- Mona Lisa N.V. as a member of the EurimGroup and manufacturer of the IUDs is certified according to the ISO 13485:2016 quality management system, which is important for medical device manufacturers. All products carry the internationally valid CE mark.
- EurimPharm's product range includes all common forms of copper IUDs which are established in the market.
General
Patients looking for safe and long-term precautions without hormones will decide for a copper-IUD. Up to now copper-IUDs are still the most common way of contraception worldwide.
The main concern of EurimPharm is to sell high quality products at lower prices than equivalent original products. The product range covers all main versions of copper-IUDs which have been proven and well known for years in various markets. EurimPharm products are defined as being similar or identical to the original products like "Multiload Cu 375/375 SL" by Organon, "Nova T 380" by Schering, "Flexi-T 300" by Prosan or as replacements of well known but no more available products like the "Gyne-T" from Janssen Cilag.
The body of the IUD is made of polyethylene with copper wire wound around the stem. The addition of barium sulphate enables radiological proofing. The total copper surface area varies between 300 mm² and 380 mm². The higher the copper surface the longer the in-situ-time within the patient´s body (from 5-10 years). The copper has a purity level of 99,99 %. The return thread at the bottom end of the body is composed of tear-proof polyamide nylon 6.
Multi-Safe CU 375 / CU 375 short
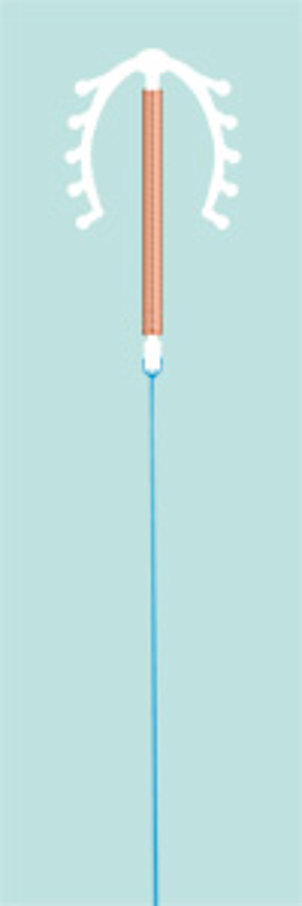
This model is completely identical with the long-term tried and trusted bestseller "Multiload Cu 375/375 SL".
Insertion requires a one-hand technique only without preloading.
This EurimPharm model is available in two versions:
Multi-Safe CU 375
With a copper surface of 375 mm² this version guarantees 5 years of safe contraception.
Unique Features:
- One-hand technique for insertion
- low expulsion rate due to the flexible IUD-body
Multi-Safe CU 375 short
This version is identical to the Multi-Safe CU 375, only differing in the dimension.
Unique Features:
- the stem length (29,4 mm) is 5 mm shorter compared to the Multi-Safe CU 375
- ideally suited for patients with shorter uterus length
T-Safe CU 380A
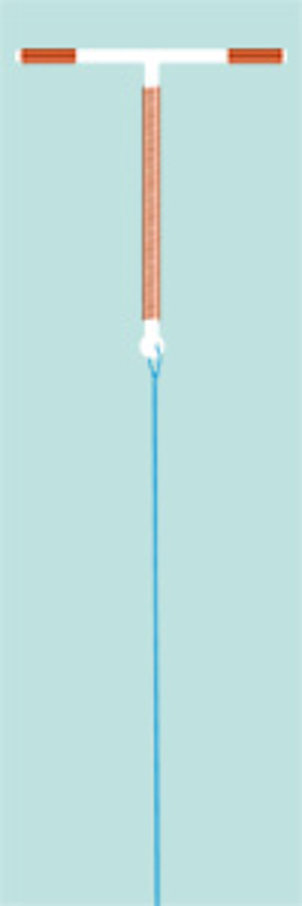
This model is not only a rebuilt but even an improvement of the former, no more available "Gyne-T".
The classic T-shaped IUD is the most common used IUD-model in the world, recommended even by the WHO. It convinces through secure fit in the uterus, lowest Pearl-Index and a minimal expulsion rate.
Before insertion, the IUD has to be preloaded, which means that the horizontal arms have to be bended into the tube. For easy loading a loading capsule is enclosed.
Unique features:
- The T-Safe CU 380A has a copper surface of 380 mm² and guarantees safe contraception up to 10 years. (Recommendation of the WHO)
- The copper sleeves are completely embedded in the horizontal arms. This results in a very smooth and plane surface as well as in a small diameter of the tube (4.4 mm)
- Small dimension of the loaded IUD with easy insertion
- Easy handling through loading capsule
T-Safe CU 380A QL
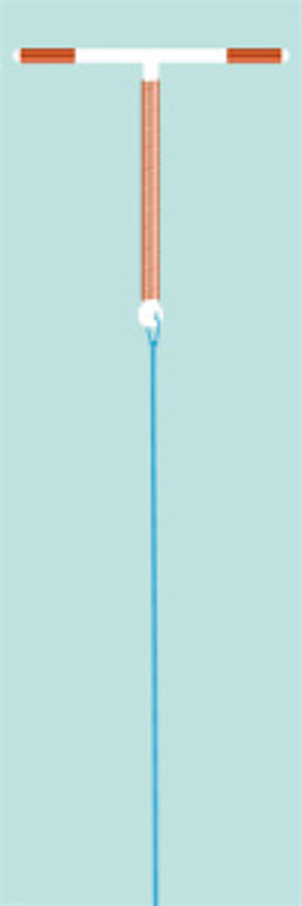
This model is an improved rebuilt of the former, no more available “GyneT” with a new technology for quick and safe loading of the IUD.
The classic T-shaped IUD is the most common used IUD-model in the world, recommended even by the WHO. With a copper surface of 380 mm² it shows the lowest pearl index among all copper IUDs.
T-Safe CU 380A QL convinces through secure fit in the uterus and in consequence low expulsion rate because of optimal memory test results.
Before insertion, the IUD has to be preloaded by using the plunger. Due to the development of the insertion body, loading of the IUD is possible in seconds.
Unique features:
- The T-Safe CU 380A QL has a copper surface of 380 mm² and guarantees safe contraception up to 10 years (recommendation of the WHO)
- The copper sleeves are completely embedded in the horizontal arms. This results in a very smooth and plane surface
- The longer insertion tube of the T-Safe CU 380A QL improves positioning of the IUD with patients with a longer uterine cavity
- Effortless and quick loading of the IUD because of the new insertion body
Neo-Safe T CU 380 / 380 Mini

This EurimPharm model has the same design as the "Nova T 380".
Also this model requires preloading before insertion. In contrast to the T-Safe CU 380A version, the horizontal arms are to be pulled into the tube.
This EurimPharm model is available in two versions:
Neo-Safe CU 380
With a copper surface of 380 mm², this IUD model guarantees safety up to 5 years.
Unique features:
- Simple handling
- Easy and painless insertion and removal
- Low PID (Pelvic inflammatory disease) rate
Neo-Safe CU 380 Mini
The Neo-Safe CU 380 Mini has identical features like the Neo-Safe CU 380 and differs only in the dimension from the standard model.
Unique features:
- less stem length as well as less width of the side arms compared to the Neo-Safe CU 380
- ideally suited for women with a uterus length < 7 cm
Distribution Network
Made in Europe - Available worldwide
EurimPharm IUDs are distributed in Europe by local national distribution partners.
The partners are selected according to EurimPharm´s quality management principles which also rule all further cooperation. New distribution partners have been acquired worldwide in recent years, so that EurimPharm IUDs are currently available in more than 25 countries (partly under other brand names).
Please do not hesitate to contact us if you are interested in a partnership, if you have any questions about our products, or if you would like to know more about our distribution partners.
Quality System
All EurimPharm IUDs meet the required international standards according to Council Directive 93/42/EEC Annex II.
Mona Lisa N.V., as a member of the EurimGroup and manufacturer of the IUDs, is certified according to the quality management systems ISO 13485:2016 and CAN/CSA ISO 13485:2003. All products carry the internationally valid CE mark.
EurimPharm IUDs are manufactured in Belgium in sterile class 10,000 (ISO 7) production facilities. Of course, these premises are subject to permanent quality inspections.
All valid certificates can be downloaded for viewing under the corresponding link.
How does an IUD work?
However, nowadays it is assumed that the most probable effect is that it disrupts the normal function of the male gametes (sperm), which become incapable of fertilizing the female egg. It is also assumed that copper ions influence the development of the egg so that fertilization doesn´t take place: IUDs are no longer considered a method of abortion.
When should an IUD be inserted?
Insertion is recommended during or shortly following menstruation. If pregnancy is excluded, EurimPharm IUDs may be inserted at any time of the cycle. Postpartum insertion should be postponed until six weeks after delivery, as this is associated with high rates of perforation and expulsion. There are no doubts against breast feeding at lying IUD.
Are there interactions with other drugs?
<p> </p>
<p>The available experience indicates that, in general, drugs interfering with contraceptive efficacy of Mona Lisa IUDs are highly unlikely. However, published reports appear to show diminished efficacy in the presence of long-term use of non-steroidal anti-inflammatory drugs (especially acetylsalicylic acid) and corticoids. Short-term use in the treatment of dysmenorrhea with not-steroidal anti-inflammatory drugs does not appear to reduce contraceptive efficacy.</p>
<p> </p>
When may EurimPharm IUDs not be used?
EurimPharm IUDs may not be used at:
- Malignant diseases of the genital tract
- Vaginal bleeding
- Pregnancy
- Past history of ectopic pregnancy or predisposing factors
- infections of the genital tract
- Sexually transmitted diseases during the last 12 months (except bacterial vaginitis, repeated herpes infection, hepatitis B)
- Abortion with infection during the last 3 months, pelvic inflammatory disease (PID)
- Uterine malformations (congenital or acquired)
- Allergy to copper
May a magnetic resonance imaging (MRI) be performed with the IUD in place?
MR Conditional
Non-clinical testing has demonstrated that the Mona Lisa intrauterine devices are MR condition. A patient with a Mona Lisa intrauterine device can be safely scanned in a MR system with a static magnetic field up to 3 Tesla.
The signed Declaration of the manufacturer is available under section "Quality System".

